
“Organic chemistry is not just a subject, it’s a way of life.” – Anonymous
Have you ever wondered how atoms and molecules undergo transformations? How one group can be substituted for another in a chemical reaction? If so, you’re about to embark on an exciting journey into the world of electrophilic substitution reactions.
In organic chemistry, electrophilic substitution is a key concept that involves the replacement of an atom or group in a molecule by an electrophile. This process commonly occurs in aromatic compounds, where substituent groups are added or replaced to create new compounds with unique properties.
An electrophilic substitution reaction is a type of reaction in which a functional group is replaced by an electrophile. In this reaction, hydrogen from the compound is replaced by an electrophile.
An electrophile is an electron-deficient species or electron-loving. Electro means electron and phile mean loving. The general reaction of E.S Rx is given:
A-B + E+ → A-E + B+
Is it necessary for every nucleophile to have a positive charge? The answer is no. Benzene and its derivatives (nitrobenzene, toluene, benzaldehyde, phenol, etc.) give electrophilic substitution reactions. Benzene has pi electron-rich species and pi electrons are loosely bounded due to these weakly bounded pi-electrons, the electrophile attracted toward benzene. Generally, the compounds having double bond gives an addition reaction but why benzene gives a substitution reaction?
Benzene gives a substitution reaction not an addition reaction because when benzene undergoes an addition reaction, the product formed is non-aromatic. When benzene gives a substitution reaction, the product remains aromatic and in chemistry, aromatic compounds are more stable. Benzene gives an addition reaction but at critical conditions (at high temperature, pressure, catalyst) but generally, benzene and its derivatives give a substitution reaction.
To understand this phenomenon better, let’s take a closer look at the reaction energy diagram. During electrophilic substitution, reactivity plays a crucial role as it determines the ease with which a substituent group can be added or replaced. The activation energy required for this process depends on various factors such as the nature of the reagent and the functional groups involved.
Mechanism of Electrophilic Substitution Reaction
The mechanism of electrophilic substitution reactions involves a stepwise process that leads to the formation of a carbocation intermediate. This reaction occurs when an electrophile attacks an aromatic ring, resulting in the substitution of one group for another. Let’s delve into the details of this mechanism.
Nucleophilic Attack by the Aromatic Ring
In the first step of the mechanism, an electrophilic species (the electrophile) initiates the reaction by attacking the electron-rich aromatic ring. The electrophile is attracted to the π electrons present in the ring due to their high electron density. This attack causes a temporary disruption in the aromaticity, leading to the formation of a positively charged intermediate known as a carbocation.
Formation of Carbocation Intermediate
The carbocation intermediate is formed when one of the carbon atoms in the aromatic ring loses its bonding electron pair and becomes positively charged. This positive charge is stabilized by neighboring groups through resonance or conjugation effects. The stability of this intermediate greatly influences both the reaction rate and product selectivity.
Nucleophilic Attack on Carbocation Intermediate
Once formed, the carbocation intermediate can be attacked by a nucleophile, which is typically part of another molecule or ion. The nucleophile donates its pair of electrons to form a new bond with the positively charged carbon atom in the carbocation. This nucleophilic attack results in substitution, where one group is replaced by another on the aromatic ring.
Addition-Elimination or Elimination-Addition Mechanism
Electrophilic substitution reactions can proceed via two different mechanisms: addition-elimination or elimination-addition. In an addition-elimination mechanism, after nucleophilic attack occurs, an additional step involving elimination takes place. This elimination step restores aromaticity and generates a new compound with substituents attached to it. On the other hand, in an elimination-addition mechanism, the elimination step occurs first, followed by the addition of a nucleophile to the carbocation intermediate.
Influence of Electron-Withdrawing Groups
The presence of electron-withdrawing groups on the aromatic ring can significantly affect the reaction rate and product selectivity in electrophilic substitution reactions. These groups have a strong electron-withdrawing effect due to their ability to withdraw electron density from the ring through resonance or inductive effects. This leads to increased reactivity towards electrophiles and alters the regioselectivity of the reaction.
Understanding the mechanism of electrophilic substitution reactions is crucial for predicting and manipulating chemical reactions involving aromatic compounds. The stepwise process involving formation of a carbocation intermediate, nucleophilic attack by the aromatic ring, and subsequent substitution provides insights into how these reactions occur. Being aware of different mechanisms and factors that influence reaction outcomes allows chemists to design more efficient synthetic routes and develop new molecules with desired properties.
Types of Electrophilic Substitution Reactions
- Aromatic substitution reactions
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reactions
- Aliphatic substitution reactions
- Radical substitution reactions
In the world of organic chemistry, there are various types of electrophilic substitution reactions that take place. These reactions involve the replacement of a hydrogen atom in an aromatic compound with another functional group. Let’s explore some common types:
Halogenation
Halogenation is a type of electrophilic substitution reaction where a hydrogen atom in an aromatic ring is replaced by a halogen atom, such as chlorine or bromine. This process occurs due to the high reactivity of halogens and their ability to act as strong electrophiles. Chlorination and bromination are two examples of halogenation reactions.
- Pros:
- Halogenation can be utilized to introduce different halogens into aromatic compounds, allowing for the synthesis of diverse products.
- It is a relatively straightforward reaction that can be carried out under mild conditions.
Cons:
- The use of certain halogens, such as fluorine, can lead to unwanted side reactions or difficulties in achieving selectivity.
- The reaction may require the presence of a catalyst or specific reaction conditions.
Nitration
Nitration involves the introduction of a nitro group (-NO2) into an aromatic ring through an electrophilic substitution reaction. This process typically utilizes nitric acid as the nitronium ion (NO2+) donor, which acts as the electrophile. Nitration is commonly employed for the synthesis of compounds like nitrobenzene and TNT (trinitrotoluene).
Pros:
- Nitration allows for the incorporation of nitrogen-containing groups into aromatic compounds, expanding their range of applications.
- It offers good control over regioselectivity, enabling precise placement of the nitro group on specific positions within the aromatic ring.
Cons:
- The use of strong acids like nitric acid can pose safety hazards and requires careful handling.
- Nitration reactions may sometimes yield unwanted by-products or result in mixtures of regioisomers.
Sulfonation
Sulfonation is a type of electrophilic substitution reaction where a sulfonic acid group (-SO3H) is added to an aromatic ring. This process involves the reaction of an aromatic compound with sulfur trioxide (SO3), which acts as the electrophile. Sulfonation reactions are commonly employed in the production of dyes, detergents, and pharmaceutical intermediates.
Pros:
- Sulfonation allows for the introduction of highly polar sulfonic acid groups, which can enhance solubility and reactivity.
- It offers good control over regioselectivity, allowing selective placement of the sulfonic acid group on specific positions within the aromatic ring.
Cons:
- The use of sulfur trioxide can be challenging due to its corrosive nature and potential hazards.
- Sulfonation reactions may require careful temperature control to achieve desired selectivity and prevent over-sulfonation.
Steps in Electrophilic Substitution Reaction
Electrophilic substitution reaction generally proceeds by the mechanism which contains three steps:
- Generation of Electrophile
- Attack of Electrophile
- Removal of old Electrophile
Now we discussed a particular example with a detailed mechanism.
Halogenation (Chlorination) of Benzene

Mechanism
1. Generation of Electrophile
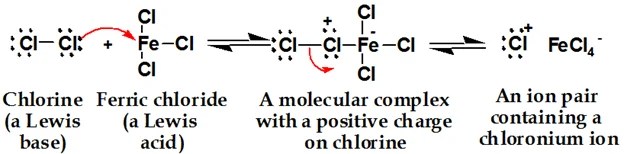
2. Attack of Electrophile

The question is that the sigma complex is not aromatic so why it is formed? It is not aromatic but it has a little bit stable due to resonance. There is no formation of a carbocation in E.S Rx of benzene. In E.S Rx of benzene, a sigma complex is formed.
3. Removal of old Electrophile

Can we use another catalyst in the halogenation of benzene except for FeCl3?
Yes, we can! FeCl3 is a lewis acid so we used another lewis acid as a catalyst like AlCl3, AlBr3, ZnCl2, etc. The purpose of the catalyst is to generate the electrophile and remove the old electrophile.
Which of the following compounds gives a more electrophilic substitution reaction? A) Benzyl cyanide B) Phenol
Properties Of Benzyl Cyanide:
The following are the properties of Benzyl Cyanide:
- They are colorless only liquids
- They have a density of 1.015 g/cm3
- They have a melting point of −24 °C (−11 °F; 249 K).
- Also, they possess a boiling point of 233 to 234 °C (451 to 453 °F; 506 to 507 K).
Properties of Phenol:
- Phenol is an aromatic compound with a benzene ring in it.
- Phenol possesses a peculiar smell.
- These are water-soluble crystalline solids that have definite shapes and volumes in the 3-dimensional pattern.
- They have low melting and high boiling point.
What is the Mesomeric Effect?
The mesomeric effect is the effect that enables a ring to become stable. It may increase or decrease the electron density of the ring. So, it may enhance the electrophilic substitution reaction. There are some compounds that are attached to the benzene ring so that they may be activated or deactivated groups. If the ring is electron deficient then the +M effect dominated and they give electrons to the ring the stabilize charge density. If the ring is electron enriched the -M effect dominated and withdraws the electron from the ring to stabilize the charge density.
Which is more stable Benzyl Cyanide or Phenol?
Structures of Benzyl Cyanide and Phenol:
The first structure shown is Benzyl chloride and the second one is phenol. Both stabilities are defined by the mesomeric effect.
The mesomeric effect contains two types of mesomeric effects that are +M effect and the -M effect. +M effect is shown by those compounds having lone pair on them and is directly attached to the benzene ring however on the other hand -M effect is shown by compounds that are directly attached to the ring and with the other compound that is more electronegative than that of compound attached to the benzene ring with pie bond.
In the structure of Phenol, oxygen contains lone pairs on it so that it gives its lone pair to the ring yet the charge density of the overall ring increases. Therefore, it gives a substitution reaction. While in the structure of Benzyl cyanide there is no increase in charge density because carbon takes an electron from the benzene ring and nitrogen takes an electron from carbon so electronic charge density decreases.
By the following relation, it is clear that (Electron charge density is directly proportional to substitution reaction). Therefore, the increase in electronic charge density in phenol gives more substitution reactions.
So, B > A (Conclusion of the question)
Frequently Asked Questions
What are electrophilic substitution reactions give example?
An electrophilic substitution reaction occurs when an electrophile (an electron pair acceptor) replaces the functional group connected to a compound. Alkylation, acylation, halogenations, nitration, sulphonation, and other processes are electrophilic substitution reactions of benzene
What are 5 electrophilic substitution reactions?
- Alkylation
- Acylation
- Halogenations
- Nitration
- Sulphonation
What are electrophilic and nucleophilic substitution reactions?
Nucleophilic substitution reaction:
It is a chemical reaction in which a nucleophile displaces a leaving group. The donor of the electron is a nucleophile.
Electrophilic substitution reaction:
It is a chemical reaction in which an electrophile displaces a functional group. Electrophiles take electrons in.
What is the four most common electrophilic aromatic substitution reaction?
Nitration:
Nitration is the process of replacing H with NO2, utilising sulphuric acid (H2SO4) as the Lewis acid and nitric acid (HNO3) as the source of NO2:
Sulfonation:
Sulfur trioxide (SO3) can be used to execute it when sulphuric acid (H2SO4) is present as the Lewis acid:
Friedel-Crafts Alkylation:
We first add a Lewis acid like AlCl3 or FeCl3 to an alkyl halide “R-X” such as CH3CH2Cl. Similar to Cl2, the Lewis acid quickens the reaction by coordinating with the halogen, weakening the C-Cl bond, improving its leaving group properties, and facilitating the nucleophilic attack on the attached carbon.
Friedel-Crafts Acylation:
With an acyl halide, we begin. The addition of our Lewis acid causes the breakdown of C-H and the production of C-C.
Why benzene is an electrophilic substitution?
The benzene ring’s resonance causes the delocalized electron to traverse the carbon atoms there effectively. Along with stabilizing the arenium ion somewhat. Because of the arenium ion’s partial stability, electrophilic substitution reactions in benzene are very common.









Leave a Reply