
Hydrosulfuric acid is also called Hydrogen Sulfide or sewer gas. It has the chemical formula of H2S. It is colorless and has an odor of rotten eggs. It is poisonous, corrosive, and flammable. It is manufactured when organic matter is breakdown microbially in the absence of oxygen. This process is called anaerobic digestion, which is done without oxygen.
Hydrogen sulfide concentration in ambient air ranges from 0.11-0.33 ppb (parts per billion), but it ranges up to <1 ppb in urban areas. However, Much large quantity has been detected near local communities. Hydrosulfuric acid readily evaporates from the water’s surface and has no concentration in soil. Hydrogen sulfide or hydrosulfuric acid and its metabolites eliminate from the body with urine or with feces.
It plays a vital role in redox biology as an antioxidant & signaling molecule to support cellular function. Carl Wilhelm Scheele discovered the chemical composition of hydrosulfuric acid in 1777.
Properties Of Hydrosulfuric Acid
Hydrosulfuric acid may possess many the properties such as Physical properties, Structural properties, Thermochemistry properties, and chemical properties. These properties are changes in the physical and chemical state of matter.
1. Physical properties
Physical properties may include changes in the physical state of matter. So, Physical properties possess chemical formula, Molar mass, appearance, odor, melting point, boiling, density, etc.
- The Chemical formula of Hydrosulfuric acid is H2S.
- It possesses a molar or molecular mass of 34.08 g/mol.
- They are colorless liquids as well as gas.
- The meting point of hydrosulfuric acid is -82℃.
- The boiling point of hydrosulfuric acid is -60℃.
- At 4g/dm3, hydrosulfuric acid is soluble in water.
- Its density is 1.363 g/dm3.
- Hydrosulfuric acid possesses a vapor pressure of 1749kPa
- Normally it is a neutral species and has a pKa value equal to 7.
- Its Refractive index is 1.000644 at 0℃.
2. Thermochemistry
Thermochemistry includes specific heat capacity, standard molar entropy, and standard entropy for motion.
- Hydrosulfuric acid has a specific heat capacity of 1.003 J/Kg
- It has the standard molar entropy of 206 J/mol K
- Its standard entropy for motion is -21 KJ/mol.
3. Chemical Properties / Chemical reactivity
A chemical change in the states of matter or interaction with it is called chemical properties or chemical reactivity. Following are the chemical properties of the chemical reactivity of hydrosulfuric acid.
- The weight of hydrosulfuric acid is higher than that of the air. H2S is utilized to manufacture sulfur dioxide and water in the presence of oxygen with a blue flame. Especially, in the presence of base H2S act as a reducing agent.
- Hydrogen sulfide and its solution are colorless. It is soluble in water. When it reacts with the air it oxidizes to form elemental sulfur which is insoluble in water.
- Metal sulfides are often dark-colored solids that are insoluble in water they form when H2S reacts with the metal ion. H2S is also responsible for tarnishing metals like silver and copper.
- At 90 GPa pressure, H2S acts as a metallic conductor.
Manufacturing of Hydrosulfuric acid
Hydrosulfuric acid is obtained by separation from sour gas. It is prepared by the following methods:
- We ca prepare hydrosulfuric acid by treating hydrogen with the elemental sulfur at 450℃.
2H2 + 2S → 2H2S
- Hydrosulfuric acid is also prepared by laboratory method when we react ferrous sulfide with strong acid in the Kipps generator.
FeS + 2HCl → FeCl2 + H2S
- Thioacetamide is often used to generate Hydrosulfuric acid when thioacetamide is reacted with water.
CH3C(S)NH2 + H2O → CH3C(O)NH2 + H2S
- Hydrosulfuric acid is also prepared by metal or non-metal that react with water to form H2S.
6H2O + Al2S3 → 3H2S + 2Al(OH)3
- Hydrosulfuric acid is also produced by heating sulfur with solid organic compounds.
Removal of H2S
The two processes can remove H2S, and it can be removed by the water and can be removed from the fuel gases.
1. Removal from water
Hydrosulfuric acid can be removed by water in different ways. Removal of water can be done by continuous chlorination, aeration, and nitrate addition.
- Continuous Chlorination is the process that is used to clean water from impurities. When we add chlorine to water it reacts with H2S acid. So, H2S acid may remove from the water.
- Aeration is the second process. If there is a concentration of H2S less than 2 mg/l we use aeration as an ideal and exemplary treatment. In this process, oxygen is added to water and a reaction occurs between water and H2S to liberate it.
- The third step is nitrate addition. Any nitrate of calcium can be used to remove hydrosulfuric acid from water.
2. Removal from fuel gas
Hydrosulfuric acid is an abundant and essential raw material in methane and biogas. The amine gas treating method is effective to remove it from fuel gas. In this process, hydrogen sulfide is first converted into ammonium salt, and the bisulfite ions are regenerated by the heating of the amine sulfide solution. By using the Claus process, hydrosulfuric acid is converted into elemental sulfur.
Lewis Structure of H2S
Lewis structure is also called dot and cross molecular structure. To draw the Lewis structure, we must remember some of the rules:
- Select a central metal atom that is least electronegative or contains the largest size or highest atomic number.
- To draw Lewis’s structure octet of a central metal atom must be completed.
- The central metal atom should remain in its maximum covalency.
- The positive charge on the atom shows that it is a central metal atom.
- The negative charge on the atom shows that it is a corner atom.
The covalency, i.e. number of electrons shared by the atom. So, the covalency of H2 is two because it transfers two electrons and the covalency of Sulfur S is six because it shares 6 electrons with Hydrogen. So, in this regard, the central metal tom is sulfur, and the corner metal atom is Hydrogen. The Lewis diagram for hydrosulfuric acid is given below:

Hybridization of Hydrosulfuric acid
According to Drago’s rule, Hydrosulfuric acid possesses no hybridization. Drago’s rule states that:
“No hybridization takes place if there is a great energy difference or electronegativity difference between two atoms.”
So, in this regard, no hybridization occurs. To follow Drago’s rule, some necessary conditions are made:
- The central metal atom must present in the 3rd period or below it with at least one lone pair.
- The surrounding electronegativity difference will be equal to or less than 2.5.
- There should be no positive charge present on the central metal atom.
So, it is concluded that all the conditions mentioned above are satisfied in the molecule of hydrosulfuric acid. So, it possesses no hybrid structures.
Geometrical structures & polarity
According to Valence Shell electron pair repulsion theory, the molecule of hydrosulfuric acid may possess a tetrahedral structure. It possesses bent geometry, and its generic formula is AB2E2 type, and E means having a lone pair on it. It also possesses bent geometry having a bond angle of 92.1o.

Basically, according to VSEPR, lone pair and bond pair take part in determining the molecule’s geometry. So, its geometry may be linear, but due to interelectronic repulsion between two lone pairs, its geometry goes bent. To discuss its polarity, it is noticed that it is a polar compound/molecule because it has two different atoms having more significant electronegativity differences. So, these both do not cancel out their dipole moment and have a net dipole moment of 133.6 pm.
MO diagram of H2S
The molecular orbital diagram of the hydrosulfuric acid may be based on the energy levels. The lower energy levels are much more stable than, the higher energy levels, and electrons feel very good in the lower energy and stable energy levels. In the molecular orbital diagram of H2S, a heteronuclear atom, one side of the energy level is Hydrogen, and one is sulfur.
Eight electrons fill in the molecular orbits as the bonding or anti-bonding electrons. It will also tell us about the bond order and the magnetic behavior of an atom. Their orbitals are very far away because of higher energy differences and energy gaps.
In the polyatomic molecular orbitals diagram, it is noticed that:
- Atomic orbitals do not exist on the same energy levels due to higher electronegative differences.
- If two atoms or more atoms have the electrons 10 or less than from it then twisting of molecular orbitals occurs.
- Highly electronegative atoms are always stable and come in a lower energy state.
- Only those atomic orbitals are written have the ability to form a covalent bond.
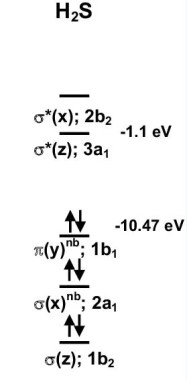
To calculate the bond angle of H2S, we use the formula:
Bond order = No. of electrons in BMO – No. of electrons is ABMO / 2
Bond order = 4 – 0 / 2
Bond order = 2
This bond order represents that H2S contains two bonds between Hydrogen and sulfur.
It also represents the presence of paired electrons. So, it is diamagnetic.
Uses of hydrosulfuric acid
- It is used to produce sulfuric acid or sulfur.
- It is also used in the manufacturing of pesticides, dyes, pharmaceuticals, etc.
- It is also used to produce heavy water for nuclear power plants.
- It is used in the field of agriculture as a disinfectant.
- Iron smelters and landfills may contain hydrosulfuric acid as a poisonous material.






