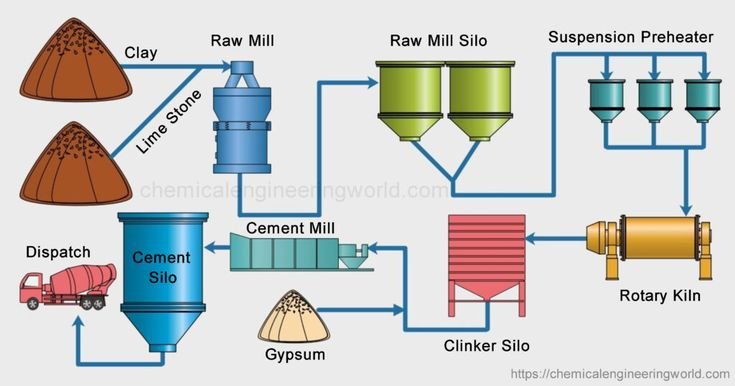
“Cement is the material obtained by burning an intimate mixture of calcarious and argillaceous materials at sufficiently high temperature to produce clinkers. These clinkers are then ground to a fine powder called cement or Portland cement.” The essential constituents are lime( obtained from limestone,e), silica, and alumina ( present in clay).
Portland Cement
An English Mason, Joseph Aspdin, introduced cement. He found it when a strongly heated mixture of limestone and clay, mixed with water and allowed to stand, hardened to a stone-like mass which resembled Portland rock, a famous building stone of England. Since then, the name Portland Cement has been given to the mixture of lime (obtained from limestone), silica, iron oxide, and alumina.
Raw Materials used for the manufacture of Cement
The important raw materials used for the manufacture of cement are:
- Calcaceous materials ( Limestone, marble, chalks, marine shells) as a source of lime ( CaO).
- Argillacous material ( clay, slate, blast furnace slag). They provide acidic compounds such as aluminate and silicates.
- Gypsum ( 2%) is used to increase the setting time.
Manufacturing Process of Cement
The manufacturing process of cement involves either a dry process. Th vhoice of dry or wet process depends on the following factors.
- Physical condition of the raw materials.
- Local climate conditions of the factory.
- The price of the fuel.
- In Pakistan, most of the factories use the wet process for the production of cement.
- Dry process needs excessive fine grinding, and it is more suited for hard materials.
- Wet process, on the other hand, is free from dust, grinding is easier and the composition of the cement can easily by controlled.
Wet Process
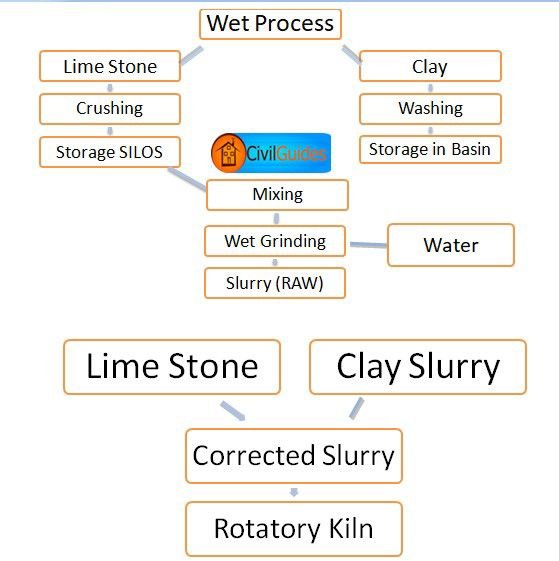
In this process it is done in the presence of water. There are five stages in the manufacture of Portland cement.
- Crushing and grinding of the raw material.
- Mixing the material in the correct proportion.
- Heating the prepared mixture in a rotary kiln.
- Grinding the heated product, known as clinker.
- Mixing and grinding of cement clinker with gypsum.
1. Crushing and Grinding
Soft raw materials are first crushed into a suitable size, often in two stages, and then ground in the presence of water, usually in rotating cylindrical ball or tube mills containing a charge of steel balls.
2. Mixing of the Raw Material
The powdered limestone is then mixed with the clay paste in proper proportion (lime 75%, clay 25%); the mixture is finely ground and made homogenous using a compressed air mixing arrangement. The resulting material is known as slurry. The slurry, which contains 35 to 45% water, is sometimes filtered to reduce the water content from 20 to 30%, and the filler cakes are stored in bins. This reduces the fuel consumption for the heating stage.
3. Heating the Slurry in a Rotary Kiln
Raw meal or sourly prepared as above is introduced into the rotary kiln with the help of a conveyor. The rotary kiln consists of a large cylinder 8 to 15 feet in diameter and 300-500 feet in length. It is made of steel and is lined inside with firebricks. The kiln rotates horizontally on its axis at the rate of 1-2 revolutions per minute, and it is inclined a few degrees. As the kiln rotates, the charge slowly moves downward due to the rotary motion.
Now the charge is heated by burning coal, oil, or natural gas. In the rotary kiln, the charge passes through the different zones of temperature where different reactions take place. The charge takes 2-3 hours to complete the journey in the kiln. The four zones in the rotary kiln are:
(a) Drying or Preheating Zone ( Minimum temperature zone)
In this zone, the temperature is kept at 500 degrees, whereby the moisture is removed and the clay is broken into Al2O3, SiO2, and Fe2O3.
(b) Decomposition Zone ( Moderate temperature zone)
Here, the temperature goes up to 900 degrees Celsius. In this zone, the limestone( CaCO3) decomposes into lime (CaO) and CO2.
(c) Burning Zone ( Maximum zone)
In this zone, the temperature goes up to 1500 degrees Celsius and the oxides, e.g, CaO, SiO2, AI2O3, and Fe2O3, combine and form calcium silicate, calcium aluminate, and calcium ferrite.
(d) Cooling zone
This is the last stage in the kiln where the charge is cooled up to 150-200 degrees clecius
4. Clinker Formation
The resulting product, as it comes out of the kiln, is known as cement clinker. This has the appearance of greenish black or grey coloured balls varying in size from small nuts to peas.
5. Grinding the Clinkers with Gypsum
The cement clinker is then air-cooled. The required amount of gypsum ( 2.0%) is first ground to a fine powder and then mixed with clinker. At this stage, finihsed cement is pumped penumatcally ( air driven) to storage silos( stores for materials in bulk) from where it is drwan for packing on paper bages or for dispatch in bulk containers.
Setting of cement
” Cement paste when combined with water and allowed to stand for sometime then the resulting mass becomes hard and very resisitant to pressure. Thi process is known as setting of cement.”
The use of cement in the construction of buildings is based on its property of setting to a hard mass. The reaction involved in the setting of cement is described as follows:
(I) Reactions Taking Place in the First 24 Hours
A short time after the cement is mixed with water, tricalcium aluninate absorbes water ( hydration) and froms a collidal gel of the composition, 3 Ca.AI2O3.6H2O( hydrated tri-calcium aluminate).This gel starts syrstiallizing slowly, reacts with gypsum to form the crystals of clcuim sulpho aluminate.
(II) Reactions Taking Place Between 1 to 7 Days
- Tricalcium silicate and tricalcium alinate get hydrolyzed to produce calcium hydroxide and aluminium hydroxide.
- The calcium hydroxide thus formed starts changing into needle-shaped crystals, which get studded in the colloidal gel and impart strength to it.
- Aluminum hydroxide, on the other hand, fills the interstices, resulting in hardening of the mass. The gel formed starts losing water partly by evaporation and sets to a hard mass.
Cement industry in Pakistan
For the time of partition in 1947, there were four cement plants in West Pakistan, which produced about 330,000 tons of cement every year. However, in 1959, the production of cement went up to 660,000 tonnes. In 1956, two more cement factories were set up at Duad Khel and Hyderabad, but even then, the production of cement was not enough to meet the increasing demand of the construction industry in the country.
Conclsuion
For the developing countries like Pakistan there is always an increasing need for cement for development projects. Efforts were thus made to build more factories. At present, there are about 22 cement factories in privates as well as in public sectors, which are manufacturing cement both by dry and wet processes. The total production of these 22 cement plants is 9,578,802 metric tons/annum.


