
Corrosion
Rust is a reddish-brown, flaky substance that forms on iron or steel when it reacts with oxygen and moisture in the air. This slow chemical process is called corrosion, and in the case of iron, it produces iron oxide (Fe₂O₃·xH₂O). Rust weakens metal structures over time, making them brittle and unsafe. It’s more than just a surface stain—it’s a visible sign of damage caused by a natural electrochemical reaction.
“Any process of chemical decay of metals due to the action of the surrounding medium is called corrosion.”
Causes of Corrosion
There are two ways in which corrosion takes place:
(I) By Gases
The simplest case of corrosion occurs when metals come into contact with gases of the atmosphere. The surface of metals becomes coated with compounds such as oxides, sulfides, and carbonates. Such compounds sometimes form a compact layer on the surface, protecting the metal from further attack, e.g., Al.
(II) By Water
The case would be different when the metal is in contact with water. The compounds formed in this case may dissolve in water, allowing the corrosion to penetrate further into the metal. Besides dissolving compounds, water also promotes electrochemical processes, which are one of the main causes of rapid corrosion.
Electrochemical Theory
Pure metals are not easily corroded, and even iron hardly gets corroded if pure. The impurities present in the metal promote corrosion. To understand why impurities accelerate the corrosion of metal, consider what happens when two different metals come in contact with one another in moist air. Suppose, for instance, Cu is brought in contact with AI. After sometime, we shall noticw that aluminum gets corrode while copper remains intact. This can be explained by the electrochemical theory.
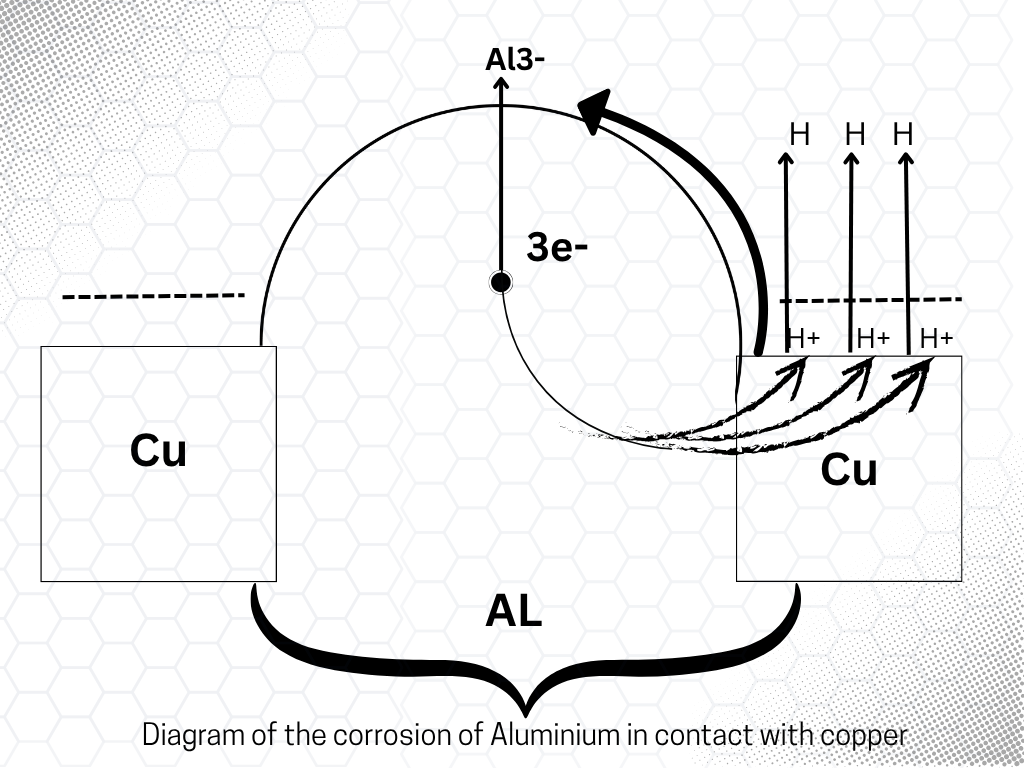
According to this theory, moisture and CO2 are present on the surface of the metal. Water ionizes into H+ and OH_ ions. CO2 dissolves in water, forming H2CO3, which ionizes as follows.
CO₂ + H₂O → H₂CO₃
H₂CO₃ ⇌ H+ + HCO₃_
This forms a galvanic cell in which Aluminum releases electrons and changes to an Al+3 ion ( being more reactive than Cu, i.e., it acts as a negative electrode and Cu acts as a positive electrode.
Anode
Al→Al3++3e− ( Oxidation)
Al3+ + 3OH_ → Al( OH)3
Aluminum ions attract OH_ ion to form Al(OH)3i.e, it starts dissolving.
Cathod
2H+ + 2e_ →H2 ( Reduction)
The H+ ion present on the Cu receives the electrons and is released as H2.
Conclusion
This is why Aluminum, higher in the electrochemical series, corrodes rapidly when in contact with copper ( lower in the electrochemical series). From this, we can conclude that when an active metal, Al ( higher in the electrochemical series), comes in contact with a less active metal, Cu( lower in the electrochemical series), a galvanic cell is established. In this process, the active metal corrodes rapidly while the other remains intact.


