
Acids are an important part of our daily life. Be it a science lab or a kitchen, acids are found everywhere. Today, we will see the types of acids, properties, uses, and a simple overview of their discovery.
What Are Acids?
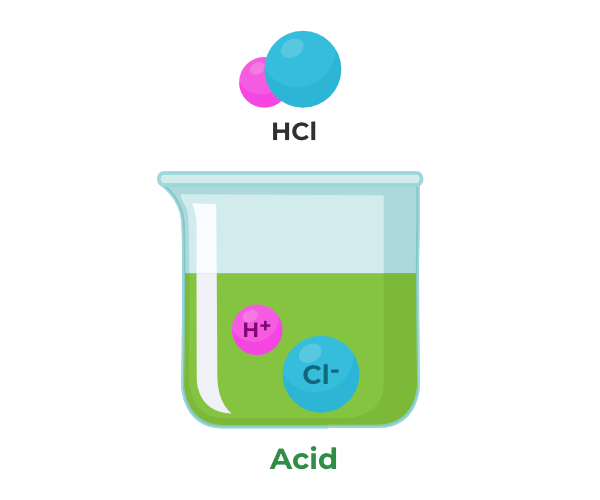
Acids are substances that, when dissolved, form H⁺ ions and release them. They have a sour taste, like lemon juice.
Examples:
- Hydrochloric Acid (HCl)
- Sulfuric Acid (H₂SO₄)
- Acetic Acid (CH₃COOH)
Discovery of Acids
The discovery of acids is quite ancient. Here are some main milestones:
- Lavoisier (1700s) linked acid to oxygen (which was later proven wrong).
- Arrhenius (1884) explained that acids release H⁺ ions in water.
- Bronsted-Lowry Theory says that an acid is a proton donor.
- Lewis theory calls acid an electron pair acceptor.
All these theories evolved from acid-base chemistry.
Properties of Acids
Here are some common properties of acids:
1. Sour Taste: Like the taste of tamarind or lemon.
2. Corrosive Nature: Strong acids and metals have the power to damage them.
3. Litmus Paper Reaction: Acids turn blue litmus into red.
4. Conduct Electricity: They conduct electricity after dissolving in water.
5. React with Metals: Acids react with metals to release hydrogen gas.
Types of Acids
We can divide acids into different categories:
1. Based on Source
- Mineral Acids—Sulphuric acid, Nitric acid
- Organic Acids—Citric acid, Acetic acid
2. Based on Strength
- Strong Acids—HCl, H₂SO₄
- Weak Acids—CH₃COOH, Citric acid
3. Based on Basicity
- Monobasic Acid—HCl (1 H⁺ ion)
- Dibasic Acid – H₂SO₄ (2 H⁺ ions)
- Tribasic Acid – H₃PO₄ (3 H⁺ ions)
Uses of Acids in Daily Life
We find acids everywhere every day:
1. Cleaning Agents: HCL is used in toilet cleaners.
2. Batteries: Sulfuric acid is found in car batteries.
3. Preservatives: Citric acid keeps packed food fresh.
4. Cooking: Vinegar contains acetic acid.
5. Medicines: Aspirin is a weak acid.
6. Fertilizers: Nitric acid fertilizers are used in banana cultivation.
7. Textile & Leather Industry: Different acids help in cloth dyeing.
Conclusion
Acids have become an important part of our lives. Their discovery formed the basis of chemistry. Whether you are a science student or a general user, understanding the uses and properties of acids is very useful.


