Aldehydes
Aldehydes are a very important part of organic chemistry. They are identified by their –CHO functional group. Aldehydes are mostly used in industrial processes—such as perfumes, medicines, plastics, and even the food industry.
Their general formula is R–CHO, where R can be either a hydrogen or a carbon chain (such as methyl, ethyl, etc.). Their structure contains a double bond (C=O) between carbon and oxygen, and this carbon also has a hydrogen directly attached to it, which makes them special.
Formaldehyde and acetaldehyde are the simplest and most commonly used examples from the aldehyde family. These are not just textbook compounds—they have a lot of real-life uses, such as in disinfectants, paints, plastics, and the pharmaceutical industry.
Next, we will learn how they are prepared and what their industrial importance is.
Preparation of Formaldehyde
Here we study the preparation of Formaldehyde and see how it is prepared from the laboratory method and the industrial method.
Laboratory Method
Formaldehyde is prepared in the laboratory by passing a mixture of methyl alcohol vapour and air over Pt asbestos or copper, or silver catalyst at 300 ℃.
CH₃OH + ½ O₂ → (Cu, 300°C) HCHO + H₂O
Prcedure
- Air is drawn through methyl alcohol with the help of a suction pump.
- Methyl alcohol is oxidized to gaseous formaldehyde, which is absorbed in water.
- The resulting mixture is called formalin.
- Formalin is a mixture of 40% formaldehyde, 8 % methyl alcohol, and 52% water.
Industrial Method
Formaldehyde is manufactured by passing a mixture of methanol vapor and air over into oxide-molbednum oxide or silver catalyst at 500 ℃.
CH₃OH + ½ O₂ → (FeO, Mo₂O₃, 500°C) HCHO + H₂O
Here is the temperature change up to 500°C, and the catalyst was FeO, Mo₂O₃.
Uses of Formaldehyde
Formaldehyde shows the following uses:
- It is used in the manufacture of resins like urea-formaldehyde and plastics such as bakelite.
- It is used in the manufacture of dyes such as indigo, para-rosaniline, etc.
- It’s 40 % aqueous solution called formaline is used as an antiseptic, a disinfectant, a germicide, a fungiside and for preserving animal specimens and sterilizing surgical instruments.
- It is used as a decolouring agent in vat dyeing.
- It is used in making the medicine urotropine, used as a urinary antiseptic.
- It is used in making formamint ( formaldehyde+lactose), used as throat lozenges.
- It is used in the processing of the anti-polio vaccine.
Preparation of Acetaldehyde
It was also prepared in in laboratory and on an industrial scale.
Laboratory Method
(a) Acetaldehyde is prepared in the laboratory by the oxidation of ethyl alcohol with acidified sodium dichromate solution.
C₂H₅OH + ½ O₂ → (Na₂Cr₂O₇+ H₂SO₄, heat) →CH₃CHO + H₂O
Procedure
- A mixture of ethyl alcohol and sodium dichromate solution is run into boiling dilute sulphuric acid.
- Immediately, a vigorous reaction takes place, and acetaldehyde formed in the liquid state is immediately distilled off.
- This prevents the oxidation of acetaldehyde to acetic acid. Ethyl alcohol remains in solution until it is oxidized.
- Pure acetaldehyde is obtained by redistillation.
(b) Acetaldehyde can also be prepared by the dry distillation of a mixture of calcium salts of formic acid and acetic acid.
Industrial Method
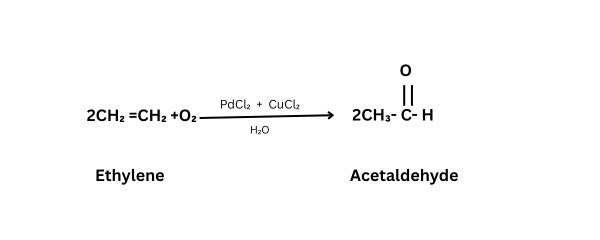
Acetaldehyde is prepared industrially by air oxidation of ethylene using a palladium chlorite catalyst with a cupric chloride promoter.
Uses of Acetaldehyde
Uses of Acetaldehyde are as follows:
- It is used in the production of acetic acid, acetic anhydride, n-butanol, ethanol, 2-ethyl-1-hexanol, vinyl acetate, paraldehyde, ethylacetate, etc.
- It is used to make acetaldehyde, which is used as a rubber accelerator.
- It is used to make chloral hydrate, ethanol trimer, and tetramer. Chloral hydrate and ethanol trimer are both used as hypnotic drugs, whereas ethanol tetramer is used as a slug poison.
- It is used as an antiseptic inhalant in nasal infections.
- It is used in the silvering of mirrors.
- It is used to make phenolic resins and synthetic drugs.


