
Alcohol
“Organic compounds containing hydroxyl as a functional group are called alcohols”. They are represented by a general formula ROH, where R is an alkyl group which may be CH₃—-, CH₃CH2, etc.
Classification of Alcohol
They are classified into Monohydric alcohols and Polyhydric alcohols.
Monohydric Alcohols
- They contain one hydroxyl group( OH).
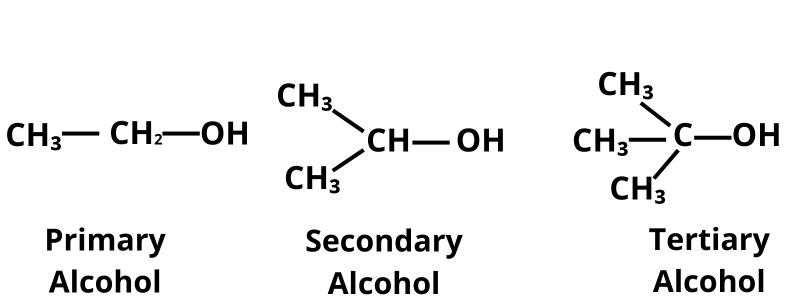
- They are further classified into primary, secondary, and tertiary alcohols.
- In Primary alcohols, the —OH functional group is attached to wth primary carbon atom, in secondary alcohols with a secondary carbon atom, and in tertiary alcohols it is attached to a tertiary carbon atom.
Polyhydric Alcohol
It was further classified into:
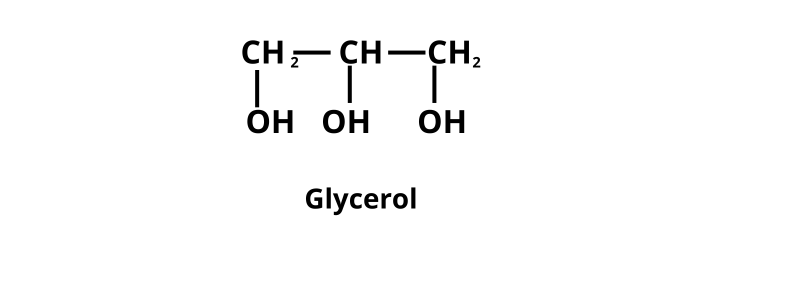
(I) Polyhydric Alcohols
They contain more than two hydroxyl groups ( —OH).
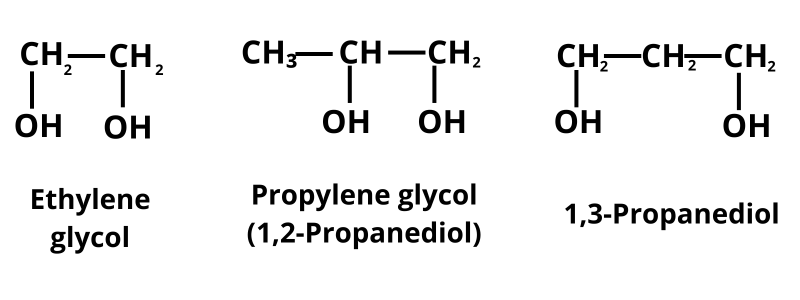
(I) Dihydric Alcohol
They contain two hydroxyl groups (—OH).
Methanol
Formally, methanol was prepared by the distillation of wood. Methanol is a simple and smallest alcohol whose formula is CH₃OH. It is a colorless and highly flammable liquid. Its smell is slightly sweet, but it is very toxic. Earlier, it was made by heating wood; hence, it is also called wood alcohol. The structure of methanol is made up of just one carbon atom, three hydrogen and one OH group. Its physical properties are typical of the group of alcohols, like a low boiling point and being easily evaporated.
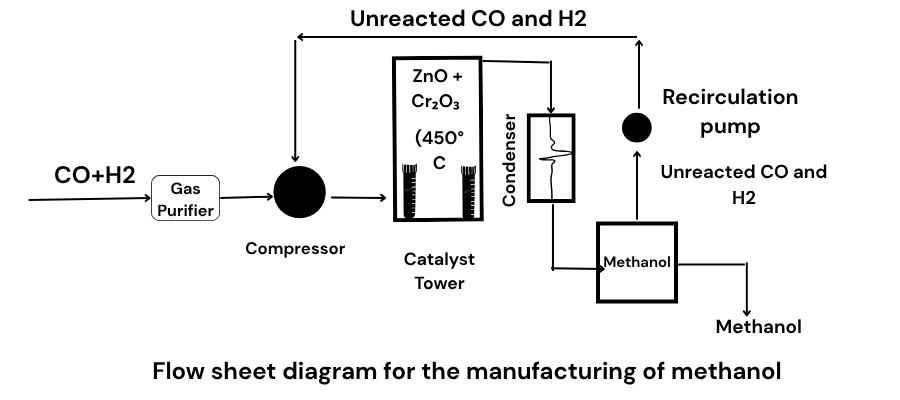
Nowadays, methanol is prepared from carbon monoxide and hydrogen or water gas a follows:
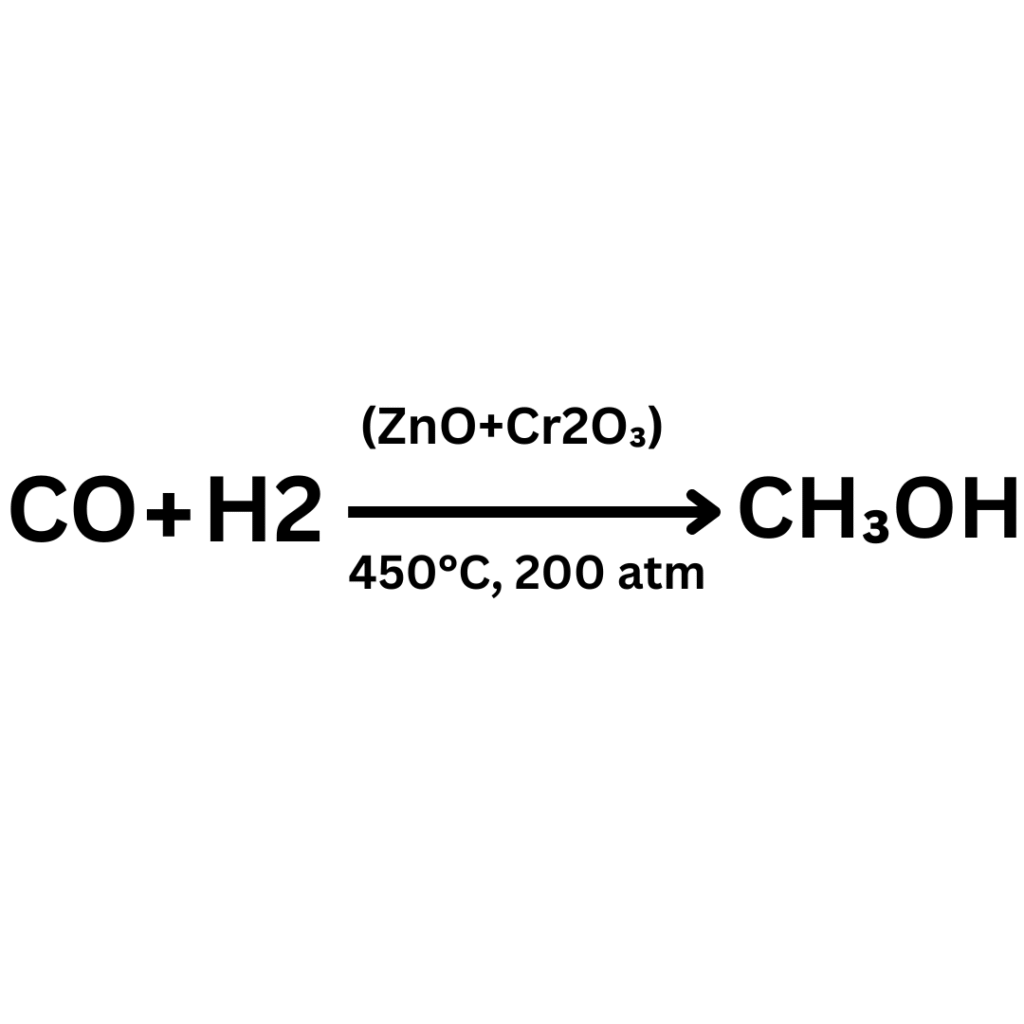
Procedure
In the first place, a mixture of carbon monoxide and hydrogen is purified. It is compressed under a pressure of 200 atmospheres and taken into a reaction chamber by means, of coild pipes. Here the cataylst is heated upto 450-500 degree. Gases react to form methanol vapours whihc are passed through a condenser to get methanol. Unreacted gases are recycled through compressor to reaction chamber.
Uses of Methanol
There are the following uses of methanol
1. As a fuel
Methanol is used as a fuel or fuel additive in vehicles and industrial burners. It burns cleanly.
2. Industrial Solvent
Methanol is used as a solvent to dissolve chemicals in paints, varnishes, and adhesives.
3. Chemical Manufacturing
Methanol is used to make chemicals such as formaldehyde, acetic acid, plastics, and synthetic fibers.
4. Antifreeze
Methanol is used as an antifreeze in cooling systems so that the liquid does not freeze even at low temperatures.
5. Laboratory Use
Methanol is also used in laboratories for cleaning and sample preservation.
6. Alternative Energy Source
Methanol is also being seen as a future fuel, like methanol fuel cells, for clean energy.
Conclusion
Methanol is a simple but powerful chemical compound that plays an important role in modern industry, fuel technology, and chemical synthesis. Be it a clean fuel or a starting material for complex compounds, the usefulness of methanol is visible everywhere. But it is equally important to understand its toxic nature and follow safety rules. Today, methanol is not just a chemical but is emerging as a smart energy alternative.


