Sodium is a soft, silvery-white metal that belongs to the alkali metals group in the periodic table. Represented by the symbol Na and having an atomic number of 11, it is one of the most abundant and essential elements on Earth. Although sodium is highly reactive and never found in its pure form in nature, its compounds—especially sodium chloride (NaCl)—are widely used in our daily lives. From playing a vital role in human health to being a key component in industries, sodium holds great significance in both chemistry and everyday applications.
Due to its high reactivity, sodium must be handled with care and is typically stored under oil or kerosene to prevent contact with air or water. In chemical reactions, it forms ionic compounds and is widely used in the production of soap, paper, glass, and various industrial chemicals. In biological systems, sodium ions help maintain fluid balance, transmit nerve impulses, and support muscle function. Its widespread presence and usefulness make sodium one of the most important elements in both science and everyday life.
Preparation of Sodium
The sodium method is prepared by Down’s method, which involves electrolysis.
Principle
Most of the sodium metal is produced by the electrolysis of fused sodium chloride. Sodium chloride is fed to the down cell. The melting point of sodium chloride is very high. 801 degrees Celsius. Some Calcium chloride is added to lower its melting point and to permit the furnace to operate at about 600 degrees Celsius.
Construction and Working
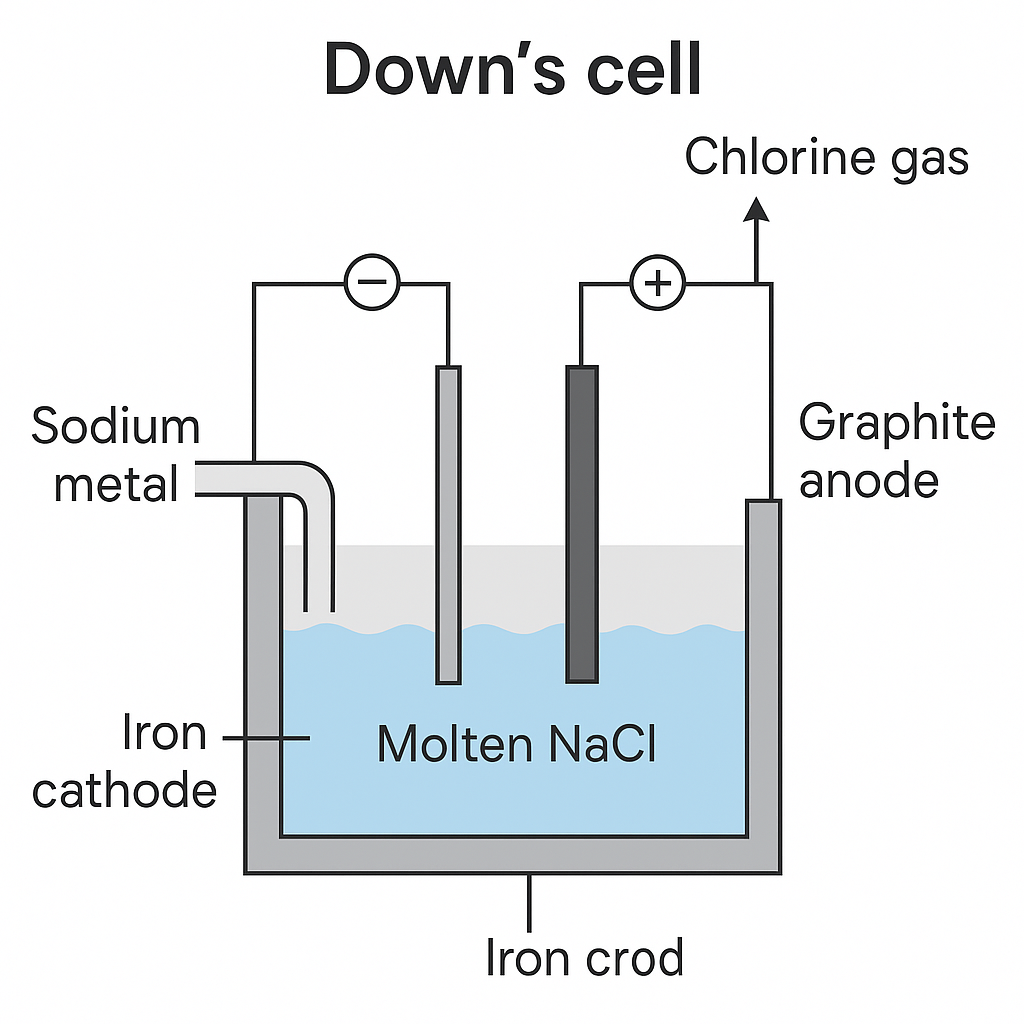
Anode
In the electrolysis cell, the large block of graphite at the centre is the anode, above which there is a dome for the collection of chlorine.
Cathode
The cathode is a circular bar of copper or iron that surrounds the anode. It is separated from the anode by a screen that is terminated in a gauze.
- The design or arrangement of Down’s cell permits the electric current to pass freely but prevents sodium and chlorine from mixing after they have been set free at the electrodes.
- When electricity is passed, Sodium metal rises in a special compartment from which it is taken from time to time. Chlorine gas is collected above the anode.
Cell Products
The cell produces dry chlorine and 99.9% pure sodium. The process is carried out at 600 degree celsius.
Reactions Taking Place During Sodium Preparation
When sodium chloride is heated at high temperatures, it ionizes as follows:
NaCl (l)⟶Na+ (l)+Cl− (l)
Na+ ions move towards cathodes and Cl- ions move towards the anode.
Reaction takes place at the Cathode ( Reduction)
The following reaction takes place at the cathode:
Na+ (l)+e−⟶Na (l)
Reaction takes place at the Anode (oxidation)
The following reaction takes place at the anode:
2Cl− (l)⟶Cl2 (g)+2e−
Net Cell Reaction
The overall reaction is:
NaCl (l)⟶Na (l)+21Cl2 (g)
Advantages of Process
There are the following advantages obtained by this process.
- The metallic fog is not produced.
- Liquid sodium can easily be collected at 600 degrees Celsius.
- Materials of the cell are not attached to the products formed during the electrolysis.
Conclusion
Down’s method is a highly practical and widely used technique for the industrial extraction of sodium. By electrolyzing molten sodium chloride, this process not only provides a continuous supply of pure sodium metal but also produces chlorine gas as a valuable by-product. Despite its high energy requirements, the method remains efficient and reliable, making it a cornerstone in the large-scale production of reactive metals like sodium.


