
Bleaching powder is a yellowish powder with a strong smell of chlorine used to disinfect water, clean surfaces, and bleach fabrics. Its chemical name is calcium Hypochlorite, Ca (OCl)Cl. When it reacts with water, bleaching powder releases chlorine, which kills bacteria and removes stains.
We use chlorine gas and dry slaked lime (calcium hydroxide) to prepare bleaching powder. The reaction takes place in special equipment under controlled conditions.
Preparation
“Typically, bleaching powder can be manufactured by the action of chlorine on dry slaked lime using one of the following methods:”
( a) Hasenclever’s method ( old method)
( b) Beckmann’s method ( modern method).
The reaction in both cases will be:
Ca(OH)₂ + Cl₂ → CaOCl₂ + H₂O
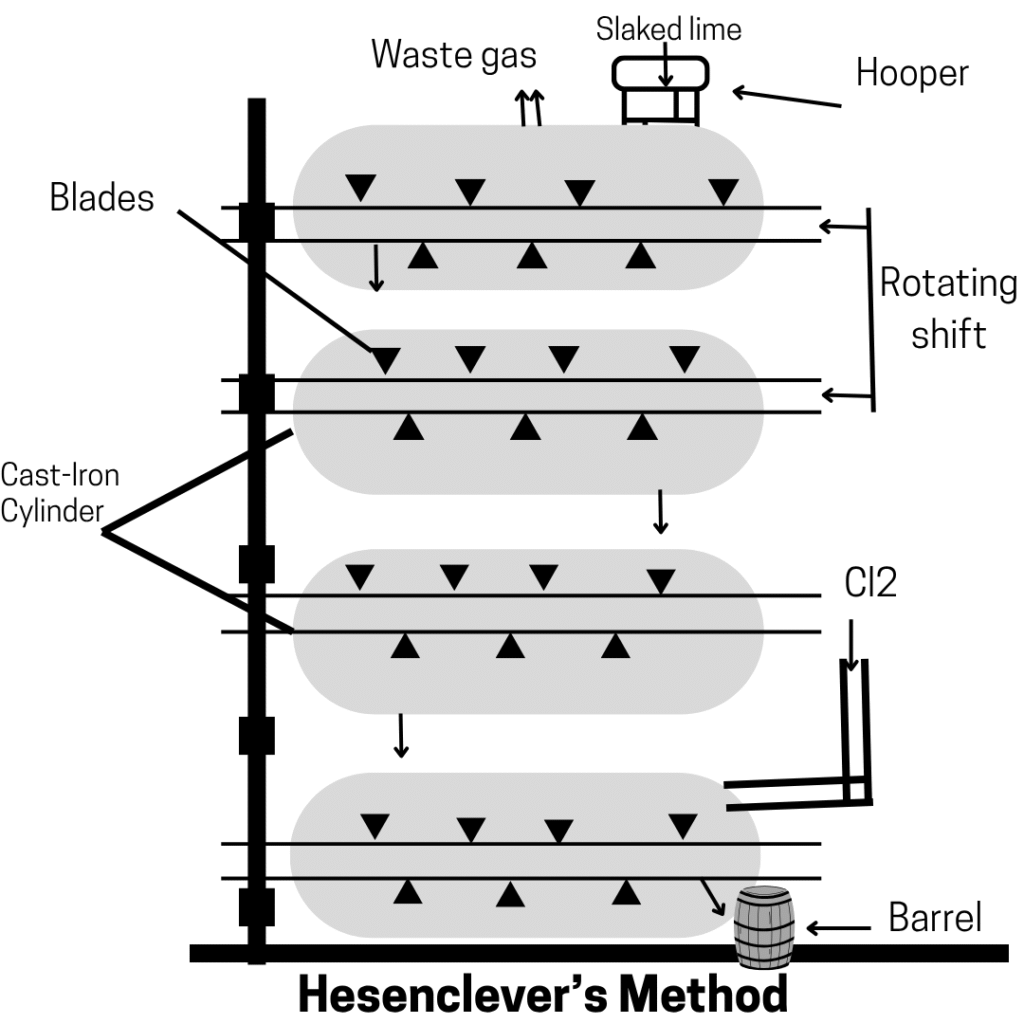
Hasenclever’s Method
- The apparatus used in this method consists of 4 to 8 iron cylinders placed one above the other horizontally.
- They are interconnected and provided with stirrers.
- The slaked lime is added through a hopper in the cylinder and is transported from one cylinder to the other with rotating stirrers.
- Chlorine introduced into the lowest cylinder rises and reacts with slaked lime to form bleaching powder, which is collected through the outlet in the lowest cylinder.
Beckmann’s Method
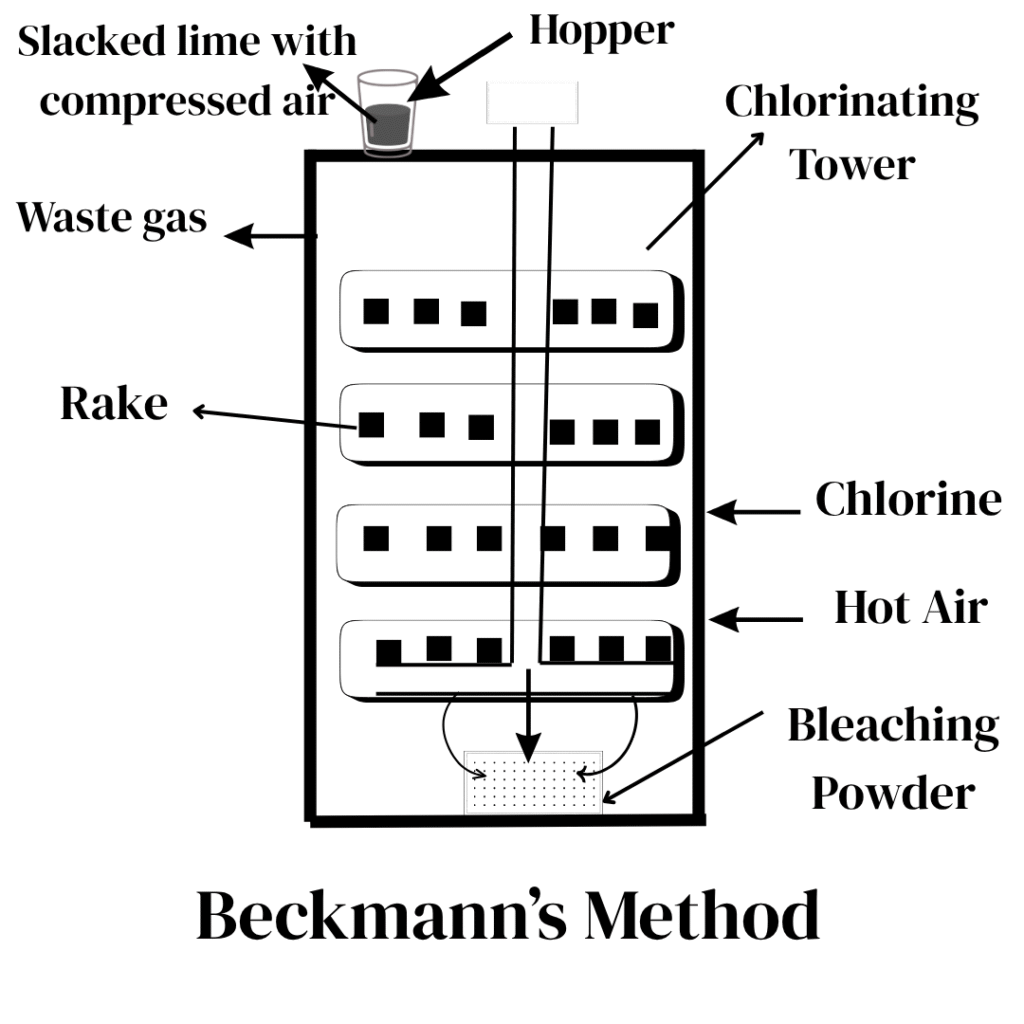
- In this method, a cast iron tower with eight horizontal shelves is used.
- On each shelf, there is a rotating rake.
- Powdered slaked lime is introduced through a hopper at the top with compressed air.
- A mixture of hot air and chlorine is introduced from the base of the tower.
- Meanwhile, slaked lime is pushed down by the rotating rakes while chlorine rises. (Blades+Stirres is called a rotating shaft)
- The apparatus works on the counter-current principle( in this principle, reacting substances move in opposite directions and intermix).
- As a result, maximum reaction of slaked lime and chlorine is brought about with very little loss of chlorine.
- Bleaching powder should always be packed in air-tight containers to avoid the loss of chlorine.
Conclusion
In conclusion, bleaching powder is not just a chemical but an essential part of everyday life. There is a scientific process and industrial planning behind its preparation. When we understand these methods, we realize how big a role this simple-looking chemical plays in public health and hygiene. Hence, its preparation is not just a chapter but an essential knowledge for every science student.


