
This blog will provide you with the general definition of meso compounds, methods to identify them, some examples, and their properties. and also, We will be able to identify a meso compound by the R and S system. This blog also contains some characteristics of meso compounds. Let’s start by having a look at their Introduction.
Introduction
Generally, a meso compound should contain two or more (not less than two) chiral centers (stereocenters). Meso compounds also have an internal plane of symmetry that divides the compound into two halves. These two halves will be mirror images and they both can reflect each other. They are super-imposable. Cyclic compounds may also be meso.
Meso compounds are also called mesomers. And their net rotation is zero.
Definition
It is a compound that has more than one chiral carbon and that is superimposable on its mirror image. They are also optically inactive.
A meso compound is an achiral compound that contains tetrahedral stereogenic centers.
Recognition
If A is a meso compound, it should have two or more stereocenters, and an internal plane, and the stereochemistry should be R and S.
- Have a look for an internal plane that lies in between the compound.
- The Stereochemistry (for example R and S) is deciding whether it is a meso compound or not. The stereochemistry of meso compounds should cancel out because we know that meso compound is optically inactive. For a moment, R cancels S out in a meso compound with two chiral centers.
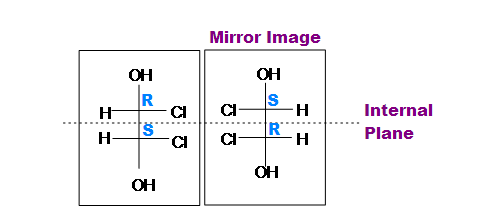
This structure contains 2 chiral centers. One is R and the other is S. First Chiral center is R and the Second is S. But, If we take a mirror image of this structure (as shown in the above diagram) then both chiral centers change from R to S and Vice versa. Also, chlorine replaces hydrogen and hydrogen replaces chlorine. And if we cut this structure from the center then we will get two equal identical halves.
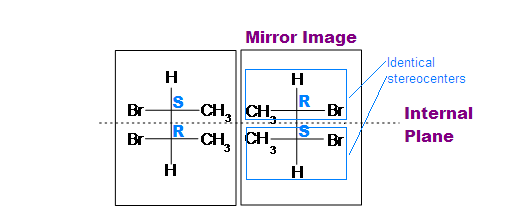
This structure also has two chiral centers. One is S and the other is R. By putting a mirror in front of this structure we get a mirror image. S chiral center will be changed to R and R will become S because of the mirror image. (by taking a mirror image bromine interchanges its position with the Ch3 group). And if we cut it from the internal plane into two halves then we get equal two parts and they will be the same.
Note: In mirror image of a compound, Dash bond will be changed to Wedge and Wedge bond will be changed to Dash bond.
Identifying a meso compound through R and S

We can identify a meso compound by the R and S system.
- In the structure of the very first compound, substituents are arranged symmetrically and stereocenters are opposite to each other. We can cut it into two equal halves. So, this is a meso compound.
- In the structure of the second compound, substituents are arranged symmetrically and stereocenters are opposite. We can cut it into two equal halves. So, this is also a meso compound.
- In the structure of the third compound, substituents are arranged symmetrically but stereocenters are the same so this is not a meso compound.
Types of Meso Compound
Meso compounds can be in many different forms such as butane, pentane, heptane and even cyclobutane.
It is not necessary for a compound to be called a meso by having only two stereocenters. Meso compound can have more than two stereocenters but not less than two.
Other Examples of Meso Compounds
Tartaric Acid
Tartaric acid is one of the best examples of meso compounds.


As we can see the mirror images of tartaric acid. There is two types of tartaric acid. One is +ve tartaric acid and other is -ve tartaric acid. There are two Chiral centers in tartaric acid (R and S).
2,3-dihydroxybutane (2,3-butanediol)
2,3-butanediol is also an example of meso compound.

If we have a look at the first structure, this structure is called (+)-2S, 3S-Butanediol because rotation is clockwise and if we look at the second structure, it is called (-)-2S, 3S-Butanediol because rotation is in anti-clockwise. And the Last structure is a Meso structure of 2,3-Butanediol.
Properties of meso compound
- Meso compounds have an internal mirror plane or a plane of symmetry.
- They have at least two chiral centers.
- They can have more than two chiral carbons but not less than two.
- Meso compounds are symmetric.
- The mirror image of a meso compound will be the same.
- We can cut a meso compound into two equal halves.
- When polarized light passes through an achiral compound, no net rotation of polarized occurs, so achiral compounds are optically inactive.
Conclusion:
Meso compounds are those compounds that have more than two chiral carbons and they are super-imposable on their mirror images like your hands on opposite sides of your body. They have the plane of symmetry and we can cut them into two equal halves they can be identified by the R and S system. Tartaric acid is the best and simplest example of meso compounds.







Leave a Reply