
Ozone layer
Have you ever wondered about the ozone layer and its crucial role in protecting our planet? The ozone layer acts as a shield, safeguarding us from harmful ultraviolet (UV) radiation. However, over the years, this protective layer has been gradually thinning, giving rise to concerns about ozone depletion and its consequences.
Human activities have played a significant role in this phenomenon. The release of substances such as chlorofluorocarbons (CFCs) into the atmosphere has led to the formation of an “ozone hole” and a decrease in ozone levels. Ozone depletion poses serious risks to both human health and the environment.
We will explore how human actions have contributed to this gradual thinning and examine the implications it has on UV radiation exposure and environmental balance.
Join us as we uncover the science behind ozone depletion and understand why preserving this vital protective barrier is crucial for our planet’s well-being.
The envelope around the earth is the ozone layer. Earth is surrounded by a layer in the stratosphere that contains a high concentration of ozone molecules known as the ozone layer. This layer protects the earth from the harmful ultraviolet radiation of the sun. This layer due to the presence of O3 molecules shows blueish color. This layer can absorb 97-99% of ultraviolet radiation from the sun. If this layer was absent or not present around the earth then harmful radiations coming from the sun cause skin diseases and weakened immune systems.
Ozone layer depletion :
Scientists have discovered a hole in the ozone layer over the Antarctic.
“ Ozone layer depletion is the gradual thinning of the ozone layer around the earth. This is due to human activities.”
This happens when chlorine and bromine atoms in the atmosphere come in contact with O3 molecules and destroy them. One chlorine can destroy 100,000 molecules of O3. It is destroyed more quickly than its form. Some substance contains chlorine and bromine when they come in contact with ultraviolet rays the chlorine present in the released and break the O3 layer. These substances are known as Ozone Depleting substances ( ODS ).

Causes of depletion :
Causes of Ozone Layer Depletion
The ozone layer, a vital shield protecting the Earth from harmful ultraviolet (UV) radiation, is being depleted at an alarming rate. This depletion is primarily caused by human activities and the release of certain chemicals into the atmosphere. Let’s explore some of the key factors contributing to ozone layer depletion.
Chlorofluorocarbons (CFCs)
This depletion is caused by no of factors. Some main factors are listed below:-
- Chlorofluorocarbon ( CFCs ) is one of the leading causes of ozone layer depletion. This is released by refrigerators, air conditioners, etc. Chlorofluorocarbons when in contact with Ultra violet rays, decomposition of CFCs occur. The decomposition of chlorofluorocarbons releases chlorine that reacts with O3 and causes the degradation of O3 molecules. This is the most major damaging factor of the O3 layer. CFCs molecule in the stratosphere is broken by UV rays. Then Chlorine release breaks O3 molecules.
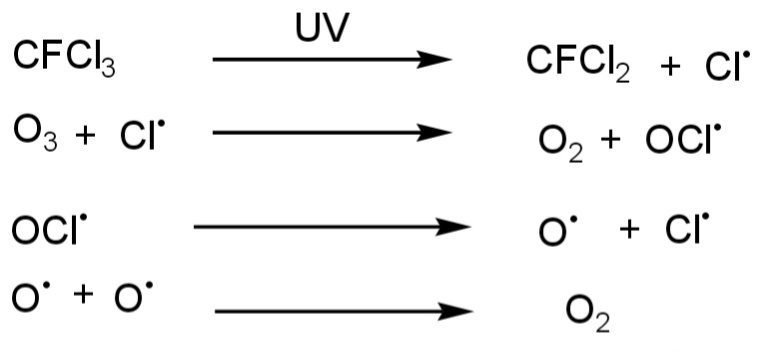
- Nitrogenous compounds are also responsible for O3 depletion. Like NO2, NO, and N2O.
- Natural causes of O3 are slightly depleted by natural processes like volcanic eruptions.
Chlorofluorocarbons, commonly known as CFCs, are one of the major culprits behind ozone depletion. These synthetic compounds contain chlorine and fluorine atoms that can break down ozone molecules in the stratosphere. Once released into the atmosphere, CFCs can remain there for several decades, continuously depleting the protective ozone layer.
Other Harmful Substances
In addition to CFCs, other substances such as halons and carbon tetrachloride also contribute to ozone destruction. Halons are typically used in fire extinguishers and can release bromine atoms that have a similar destructive effect on ozone as chlorine from CFCs. Carbon tetrachloride, once widely used as a solvent and refrigerant, has been phased out due to its detrimental impact on the ozone layer.
Industrial Processes and Aerosol Propellants
Industrial processes release significant amounts of ozone-depleting substances into the atmosphere. For example, manufacturing plants involved in electronics production or foam-blowing use chemicals that contain CFCs or other harmful compounds. Aerosol propellants found in various consumer products like hairsprays and deodorants can also contribute to ozone loss when released into the air.
Ozone depletion occurs when these harmful chemicals reach the stratosphere where they encounter UV radiation from the sun. The UV rays break apart CFC molecules, releasing chlorine atoms that then react with ozone molecules. This reaction results in a chain process where each chlorine atom can destroy thousands of ozone molecules before being deactivated.
Apart from man-made chemicals, natural processes also play a role in ozone layer depletion. For instance, nitrogen oxides released during volcanic eruptions or lightning can accelerate the breakdown of ozone in the stratosphere. However, it is important to note that the impact of human activities on ozone depletion far surpasses that of natural sources.
The consequences of ozone layer depletion are severe and wide-ranging. Increased levels of UV radiation reaching the Earth’s surface have detrimental effects on both human health and ecosystems. Prolonged exposure to UV rays can lead to skin cancer, cataracts, weakened immune systems, and other health issues. Furthermore, UV radiation affects crop yields, marine life, and terrestrial ecosystems by disrupting photosynthesis and damaging DNA.
Effects of depletion:
Harmful Effects on Health and Environment
Due to O3 depletion, many harmful effects can occur and some of them are listed below,
- Effect on Human Health Direct exposure to ultraviolet radiation may cause some serious health problems such as skin diseases, sunburns, and a weekend of the immune system.
- Effect on the environment ultraviolet rays cause minimal growth, flowering, and photosynthesis in plants.
- Effects on the buildings ultraviolet rays cause an impact on the stones of buildings and extract minerals present in them which leads to changes in color.
Ozone layer depletion has severe consequences for both human health and the environment. The increased UV radiation resulting from this depletion poses a significant threat to our well-being. Let’s explore some of the harmful effects it can have.
Increased UV radiation leads to skin cancer and eye damage in humans
Ozone depletion raises the chance of getting skin cancer from too much UV radiation. UV rays hurt the DNA in our skin cells, causing mutations that can lead to different types of skin cancer like basal cell carcinoma, squamous cell carcinoma, and deadly melanoma.
Moreover, prolonged exposure to UV radiation also puts our eyes at risk. It can lead to cataracts, a clouding of the lens that impairs vision and may require surgical intervention. It can cause photokeratitis, commonly known as “snow blindness,” which results in temporary but painful vision loss.
Ozone depletion affects air quality, leading to respiratory problems
When the ozone layer gets thinner, it lets more bad stuff into the air. This bad stuff comes from factories and people burning dirty fuels. When this bad stuff mixes with strong sun rays, it makes harmful chemicals like smog..
Breathing dirty air with lots of ozone can harm your lungs. It can make asthma and COPD worse. Even if you don’t have these problems, you might still have trouble breathing or cough from the pollution.
It disrupts ecosystems, impacting plant growth and food chains
Ozone depletion is not just bad for people, it also messes up ecosystems and puts animals and plants at risk. Ozone helps plants by blocking too much UV radiation. But when there’s less ozone, plants can’t grow as well and crops don’t produce as much.
Changes in plant growth can affect the food chain. If plants decline because of ozone depletion, animals that eat them might not have enough food. This can also affect animals that eat those animals. It could even cause some animals to die out.
Ozone-depleting substances (ODS):
Degradation of the O3 layer is caused by no of factors and each factor contains substances that chemically decompose the O3 layer. Those substances which cause ozone layer degradation are known as the Ozone depleting substances.
Some ozone-depleting substances with their sources are given below:-
- Chlorofluorocarbons released from the refrigerator, dry cleaning agent, and AC.
- Halogen was released from the fire extinguisher.
- Hydrofluorocarbon is released from the fire extinguisher and cleaning solvent.
- Methyl chloroform is released from Adhesive aerosols.
- Carbon tetrachloride released from solvent and fire extinguisher.
Solutions to Prevent Ozone Layer Depletion
Phasing out CFCs through international agreements
To stop the ozone layer from getting thinner, we need to stop using chemicals that harm it, like CFCs. The Montreal Protocol is an agreement that has helped reduce the use of CFCs all around the world. This agreement has been successful in protecting the ozone layer by stopping harmful chemicals from being released. If we follow these agreements and make sure the rules are followed, we can keep the ozone layer safe.
Promoting alternative technologies:
To help protect the ozone layer, we need to use alternative technologies that don’t harm it.
For example, we should switch to eco-friendly sprays and solvents instead of ones that damage the ozone layer. It’s also important to use air conditioners and refrigerators that don’t rely on harmful chemicals. By using these alternatives, we can help prevent ozone depletion while still enjoying modern conveniences.
Public awareness campaigns
Public awareness campaigns are important for protecting the ozone layer. They teach people about the dangers of harmful products and how to use them responsibly. By spreading the word about the negative effects of certain consumer goods, we can help people make better choices. These campaigns have been successful in changing behavior and making people more responsible for the environment.
Stop using ODS-containing products
First of all the products containing ODS should replace. Fire extinguishers that release halogen should be replaced. Use CFCs free refrigerator and Air Conditioner.
Prefer public transport
Vehicles are the worldwide source of release of greenhouse gases that causes O3 depletion as well as global warming. So prefer public transportation over personal transport. By doing so we can overcome both global warming and ozone depletion.
Cleaning products should be eco-friendly
The use of eco-friendly cleaning products will help to restrict the release of Cl and Br into the atmosphere which causes O3 layer depletion. Cl and Br are major constituents of cleaning products that find a way toward the atmosphere and cause the depletion of the O3 layer there.
Nitrous oxide should be banned
The use of nitrous oxide should be prohibited because they are the second major factor that causes O3 depletion. Like N2O, NO2, and NO.
Frequently Asked Questions
What is ozone layer depletion?
The ozone layer in the upper atmosphere of the earth is gradually getting thinner due to ozone layer depletion. The Ozone layer limits the amount of dangerous UV radiation that reaches the surface of the Earth. In the lower stratosphere (15–35 km above Earth), where the ozone layer is located, there are rather large amounts of ozone (O3).
Why is the ozone layer depleting?
The ozone layer is depleting because of manufacturing-related chemicals, particularly halocarbon refrigerants, solvents, propellants, and foam-blowing agents (such as chlorofluorocarbons (CFCs), HCFCs, and halons).
Who damages the ozone layer?
The ozone layer is being destroyed mostly by chlorofluorocarbons (CFCs), but there are other substances that harm the ozone layer as well, including those containing bromine, other halogens, and nitrogen oxides.
How can we protect the ozone layer?
- Avoid the usage of gases that are harmful to the ozone layer, such as CFCs, halogenated hydrocarbons, and methyl bromide.
- Reduce the use of automobiles.
- Avoid using cleaning products that are bad for the earth and us.
- Keep air conditioners in good working order because when they break down, CFCs escape into the atmosphere.
Is the ozone layer harmful?
Ozone is an atmospheric contaminant that harms the lungs and asthma attacks when it is present close to the ground. But ozone molecules preserve life on Earth, which is found 10 to 30 miles (16–48 km) above the planet’s surface. They aid in defending the globe against dangerous solar radiation.



Leave a Reply