
” An organo-silicon polymer in which the Si-O-Si chain is present, having alkyl or aryl groups attached to the Si-atom, is called silicomes”.
Silicon (Si), a group 14 element with atomic number 14, is a metalloid exhibiting both metallic and non-metallic properties. It is the second most abundant element in the Earth’s crust and a cornerstone of the modern electronics industry. Owing to its semiconducting properties, it is extensively used in microchips, solar panels, and various high-tech applications.
Preparation
If a compound of silicon-containing chlorine atoms and methyl group, SiCl2( CH3)2, is allowed to react with hydrogen chloride ( HCl) comes out and the silicon atoms join together through oxygen atoms.
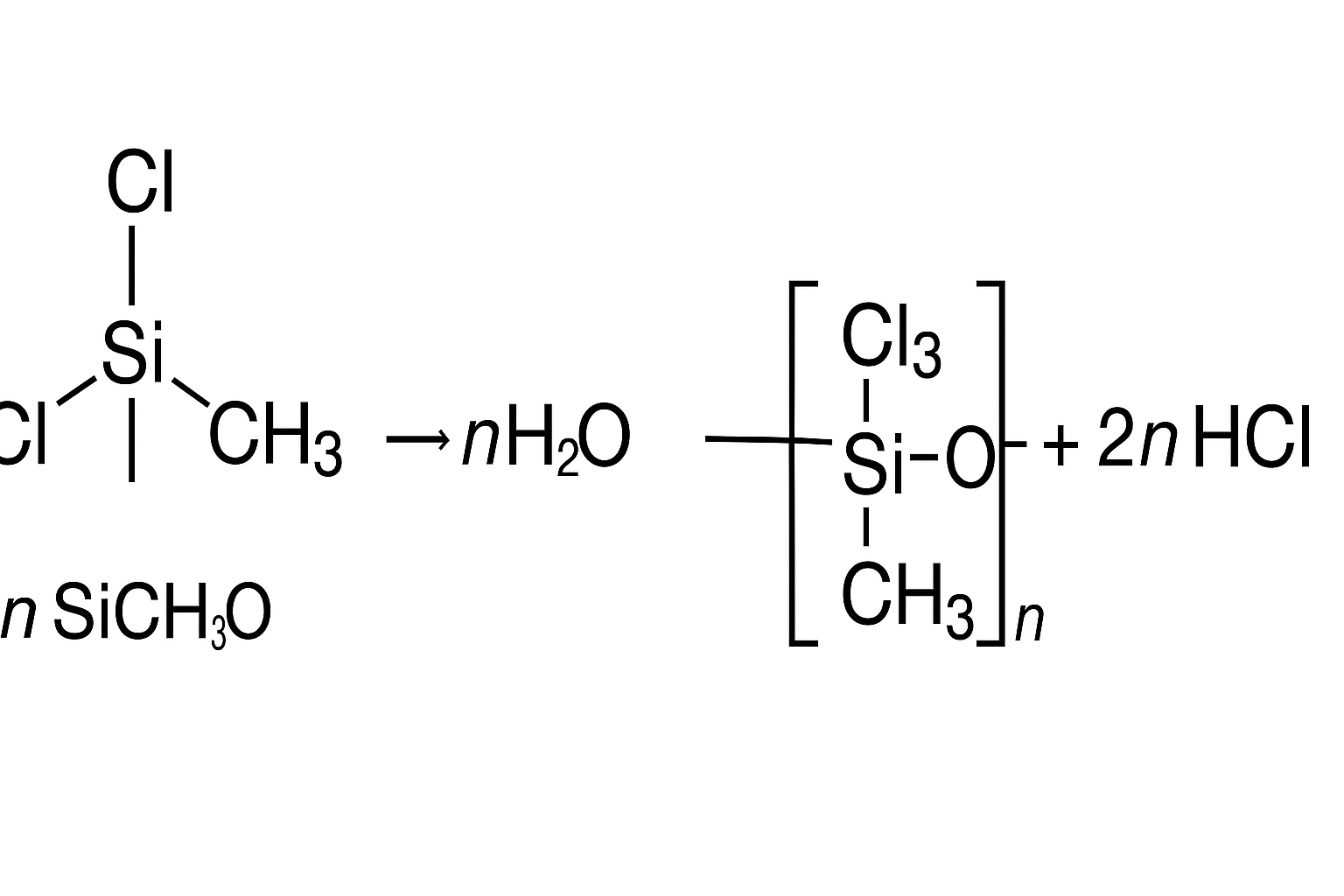
By this reaction, we can synthesize the silicon-oxygen chains found in the mineral silicates. A difference that we have CH3 groups instead of oxygen atoms joined to silicon as side chains. This type of compound is called a molecular chain cen be made of various lengths.
Occurrence of Silicon
- Silicon is very abundant, about 25% of the mass of the Earth’s crust being due to this element.
- Silicon, unlike carbon, is not found in the free state.
- It is found as a major constituent of rocks either in the form of silica or silicates.
- Most minerals other than sulphides, sulphates, phosphates, and carbonates contain a high proportion of silicon.
- As oxide, it is found as quartz in the following forms.
- Rock crystal, amethyst, quartz, smoky quartz, rose quartz, and milky quartz.
- Sand is largely silicon dioxide ( silica).
- Opal is a hydrated variety of Quartz.

Properties of Silicon
- Low Molecular mass silicons are oily liquids, while high molecular mass silicons are waxy or rubber-like solids.
- Chemical reagents do not affect them.
- They are stable towards heat.
- They are non-toxic.
- There is a small change in viscosity with the temperature change.
- They are excellent insulators.
- They are water repellent.
Uses of Silicon
There are the following uses of Silicon
1. Silicone Oils ( lubricant)
Some of the methyl siloxes are oily liquids, and they become more viscous as the chain length increases. They are used as lubricants, either incorporated in gases or as oils, in bearing gears, etc. They are also used in hydraulic brakes and other hydraulic systems.
Advantages of Silicon Oil
- The outsanding physical attrbute of silicons oil is its very small change in viscosity with change in temprature, compared with the bahoviour of other oils similar viscosity.
- If the temperature is dropped from 100 degree to 0 degree of the viscosity of petroleum oil may increase about one hundred folds, wheares that of silicons oil will increase less than four folds.
- In the presence of air or oxygen at temprature as high at 300 degree, silicone oils remains free from acid formation, oxydation and similar phenomen, which frequently limit the usefukness of petroluem products and other sunthatic organic liqiud.
2. Silicon Rubbers
Methyl silicon of high molecular mass resembles rubber and is used in making rubber-like tubing and sheets.
3. As Electrical Insulators
Silicone molecules can be made in such a way that bridges or cross-linkages bind one long molecule to another at several points along the chain. These compounds have resinous properties and are extensively used in electrical insulation.
4. Silicone Films as Water Repellents
Another interesting and important application of silicones is their use in the treatment of various surfaces to make them water repellent.
5. Silicone Film
A silicon film covers the surface and repels water like a grease film.
Uses of Silicon Film
- Much of the leakage of electricity through the moisture film on ceramic electrical insulators can be prevented by a silicon film.
- Of silicon oils that there is a small chance in viscosity with a change in temperture. Clothes, plastic, absestos, glass, leather, and paper, even filter paper and blotting paper become strongly water repllenet when covered with silicone film.
Conclusion
Silicon is more than just an abundant element—it’s the foundation of our digital age. From forming the Earth’s crust to powering semiconductors and solar cells, its unique properties make it indispensable in both nature and technology. As the demand for smarter, greener, and more efficient materials grows, silicon will continue to lead innovations that shape the future.


